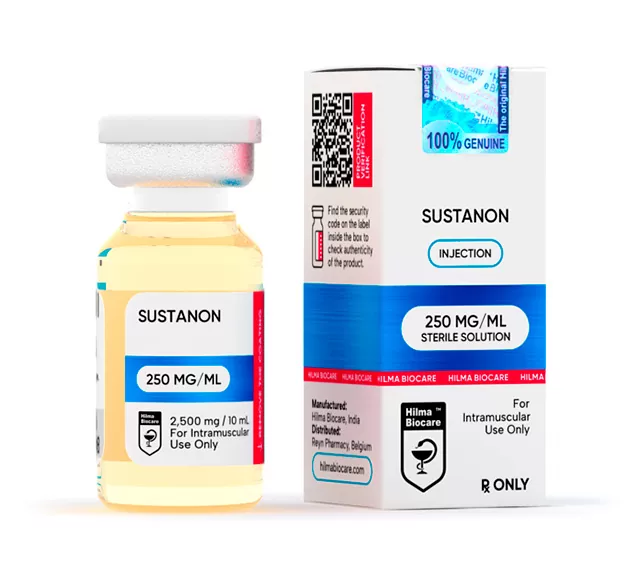
Description
Description
Specifications:
Company: Hilma Biocare
Active Half-life (Days): 15 days
Group: Anabolic Steroids
Subgroup: Injection
Dosage: 250 mg/ml
Application (Men): 250-1000 mg a week
Product pack: 10ml vial
Content (active): Testosterone decanoate 100 mg,
Testosterone isocaproate 60 mg,
Testosterone phenylpropionate 60 mg,
Testosterone propionate 30 mg
Oil-based: Yes
Retains water: No
Aromatization: No
Product Description
It has been known since the 1980s that Sustanon is a Testosterone mix comprising other four well-known Testosterone esters phenylpropionate, decanoate, isocaproate and propionate. Applied in the therapeutic practices to treat Testosterone deficiency in the body. The Sustanon performance-enhancing properties combine the best of quick and slow release Testosterones esters.
Effects:
By using Sustanon, athletes can increase muscle gains, boost strength, and lift oxygen in the blood cells to stimulate faster muscle and bone tissue regeneration. Additionally, it promotes sexual well-being, stimulates positive emotional background, and accelerates fat burn.
Side-Effects:
The most common adverse reactions attributed to the Sustanon are acne, temporary testosterone reproduction shutdown, raised Low-density cholesterol levels (LDL), and aromatization.
Protection:
You should consider medication for prolactin level regulation when using this product. Additionally, consider including gonadotropin, boldenone, letrozole, exemestane or anastrozole to minimize side effects.
Stacking:
Sustanon shows excellent results and can be safely applied with any drugs and HGH.






Reviews
There are no reviews yet.